Abstract
Background: Bendamustine (B), often administered in combination with rituximab (BR), and ibrutinib monotherapy are approved front-line therapies in chronic lymphocytic leukemia (CLL). BR is standardly administered on days 1-2 of every 28-day cycle, for up to 6 cycles. Ibrutinib is an oral inhibitor of BTK and is administered continuously until intolerance or progression. Interruption in ibrutinib treatment of ≥ 8 consecutive days has been related to inferior outcomes in CLL (Barr PM, et al. Blood 2017;129:2612-2615). The objective of this study was to examine hospitalizations of CLL patients receiving front-line treatment with bendamustine or ibrutinib in a real-world setting,
Methods: CLL patients with no prior treatment between November 2013 and December 2015 who had at least one claim for ibrutinib or bendamustine were identified from MarketScan® Commercial Claims and Medicare Supplemental databases. The index date was the first prescription for ibrutinib or bendamustine. Inclusion criteria were ≥ 18 years of age with continuous enrollment for a minimum of 6 months before and at least 30 days after the index date. Patients had variable follow-up until either the end of continuous enrollment or the end of the study period. Ibrutinib patients were stratified by number of consecutive gap days (eg, 0, 1-7, 8+). Generalized linear models were used to evaluate the rate of all-cause and CLL-specific inpatient admissions. The average length of stay for the first hospitalization occurring during the line of therapy was also modeled.
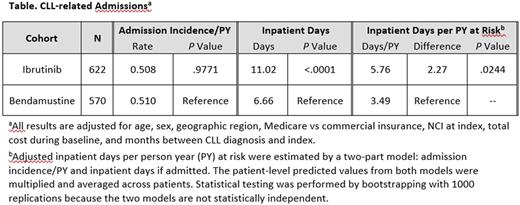
Results: This study included 1192 CLL patients receiving ibrutinib (mean 68 years of age; 66% male) or bendamustine (mean 66 years of age; 67% male) treatment; 622 patients (52.2%) had ibrutinib and 570 (47.8%) had bendamustine as front-line treatment (Table). Over 92% of the bendamustine patients received BR. Among patients with two or more prescriptions for ibrutinib (n = 560), about 48% had treatment gaps of 8 days or longer based on days' supply and fill dates on the prescription claim. The rate of hospital admissions was similar between the two groups (all cause: ibrutinib 0.57 per person years, bendamustine 0.58 per person years, P= .89; CLL related: 0.51 both groups, P= .98). Among hospitalized patients, all-cause (4.35 days longer) and CLL-related inpatient admissions (4.36 days longer) were significantly longer for ibrutinib compared with bendamustine patients (P < .0001). This effect was most pronounced in hospitalized ibrutinib patients with 8 or more gap days (all cause: 6.02 days longer, P < .0001; CLL related: 6.09 days longer, P < .0001) and to a lesser extent in the ibrutinib patients with 0-7 gap days (all cause: 2.1 days longer, P < .05; CLL related: 1.96 days longer, P= .08) compared with bendamustine patients. The analysis of ibrutinib compared with the subset of the patients with BR yielded similar results
Conclusions: Although there was no statistical difference in hospital admissions among patients receiving ibrutinib or bendamustine, ibrutinib-treated patients had significantly longer hospital stays compared with bendamustine-treated patients. In addition, nearly 50% of ibrutinib patients had gaps in treatment of 8 or more days. Nonadherence has previously been shown to be associated with clinical outcomes. More studies are needed to evaluate the consequences of treatment adherence and clinical outcomes.
Irwin: Teva Pharmaceuticals: Research Funding. Szabo: Teva Pharmaceuticals: Employment. Pathak: Teva Pharmaceuticals: Employment, Equity Ownership. Tang: Teva Pharmaceuticals: Employment.
Author notes
Asterisk with author names denotes non-ASH members.